Sharps Handling
What Exactly is a “Sharp” and Why Be Concerned?
Sharps are items capable of causing percutaneous wounds or breaks in the skin. Examples of sharps found in research laboratories include hypodermic needles, scalpel blades, razor blades, Pasteur pipettes, and broken glass. Even minor injuries from sharps can allow infectious agents to enter the body, with consequences ranging from no infection to significant disease.
It is critical to minimize the use and handling of syringes and needles, restricting their use to procedures where no alternative devices are available. Sharps injuries frequently occur due to improper handling, recapping, or disposal. Compliance with New York State Department of Health regulations is required for the certification, procurement, storage, distribution, and disposal of sharps.
Understand Risk
A thorough risk assessment is crucial for maintaining a safe laboratory environment. Consider the specific agents (e.g., human materials, viral vectors, cancer cells, pathogenic agents, biological toxins) and tools (e.g., needles, scalpels) used, the procedures performed, the working environment, and the experience level of personnel. Regularly review and update risk assessments to reflect changes in procedures, personnel, or equipment.
Reduce Risk
- Substitute: Use plasticware instead of glassware whenever possible to minimize the risk of breakage and injury.
- Limit Sharps Use: Restrict the use of sharps to situations where no alternatives are available. Consider safer options like blunt needles for non-injectable tasks (e.g., handling chemicals or drawing up solutions).
- Use Safety-Engineered Sharps: Safety-engineered sharps, such as retractable needles or those with safety covers, are designed to reduce the risk of needlestick injuries. All labs working with human materials, non-human primate materials, or bloodborne pathogens must document their consideration of safety-engineered sharps in risk assessments.
- Use Luer-Lock Syringes: Luer-lock syringes have a threaded connection between the needle and syringe to prevent the needle from slipping off. Note that these are not necessarily "safety-engineered sharps."
Best Practices
- Lock Them Up: Secure needles and syringes under lock and key when not in use, even if stored separately, as required by New York State regulations.
- Keep in Full View: Always keep sharps visible during use, and handle only one sharp at a time to prevent accidental contact or injury.
- Keep Sharps Containers Within Arm’s Reach (W.A.R.): Dispose of sharps immediately in an approved sharps container. Never bend, shear, break, recap, or manually remove needles or blades. Sharps containers should be puncture-resistant, leak-proof, and properly labeled. Replace containers when they are 3/4 full to avoid overfilling.
- Safe Transport: Use secondary containers when transporting sharps, especially in public areas, to prevent accidents and exposures.
- Proper Waste Streams: Dispose of syringes and needles in designated red sharps containers. These containers can be purchased through e-SHOP.
Things to Avoid
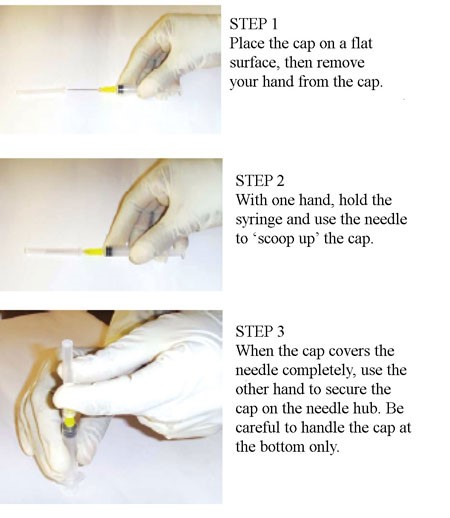
- Do Not Recap Needles: Recapping needles significantly increases the risk of needlestick injuries. If recapping is absolutely necessary, use low-cost alternatives like forceps or invest in devices like the NeedleSafe II. Obtain permission from your PI and receive training from EHS Biosafety or CARE if animals are involved. Watch instructional videos or consult training materials for proper recapping techniques.
- Alternatives to Recapping: Instead of recapping, place the sharp in a clean conical tube or directly into a sharps container if it needs to be set down temporarily.
- Do Not Use Hands to Clean Up Spills or Broken Glass: Always use appropriate tools such as forceps, tongs, or a broom and dustpan to handle sharps or broken glass safely.
- Do Not Guess: Refer to the Lab Waste Guide to determine the correct disposal method (biohazard, chemical hazard, or broken glass bin).
- Do Not Bag Sharps: Never place sharp objects in a bag; they can puncture and cause injuries.
Reporting Near Misses
Reporting near misses is essential for identifying potential hazards and preventing future incidents. All personnel are encouraged to report near misses to the Environmental Health and Safety (EHS) office through the incident reporting portal. This practice is non-punitive and aimed at enhancing overall safety in the laboratory.
Reporting and Response Procedures
In case of an injury or exposure, immediately contact your supervisor and follow your lab’s emergency procedures. Report the incident to your institution’s Environmental Health and Safety (EHS) office and document the details of the incident, including how it occurred, the type of exposure, and actions taken. Seek medical attention if necessary.
Use of Engineering Controls
Use appropriate engineering controls to minimize exposure to sharps-related injuries. Examples include working within a biosafety cabinet when handling infectious materials, using sharps disposal containers with integrated safety features, and utilizing safety shields or barriers during procedures that generate splashes or aerosols.
Institutional Resources and Support
For questions or additional support regarding sharps safety, please contact the Environment, Health and Safety (EHS) office or your designated safety representative.
More Information
- Follow the EHS Lab Waste Guide to determine disposal based on the sharps with which you work
- View the EHS Bloodborne Pathogen site if you are working with any human materials, whether or not they involve sharps handling.
- Attend Bloodborne Pathogen Training if you are working with any human materials, whether or not they involve sharps handling. This is the law.
- View the EHS Biosafety site that contains the Needle & Syringe Log, Application for Certificate of Need, and specifics on storage and use requirements for syringes and needles as required by the New York State Department of Health (DOH) Certificate of Need regulations